Possible Role Of Iron Sulfides In Creating Life In Terrestrial Hot Springs

An international team of scientists recently published a study highlighting the potential role of iron sulfides in the formation of life in early Earth’s terrestrial hot springs. According to the researchers, the sulfides may have catalyzed the reduction of gaseous carbon dioxide into prebiotic organic molecules via nonenzymatic pathways.
This work, which appeared in Nature Communications, offers new insights into Earth’s early carbon cycles and prebiotic chemical reactions, underscoring the significance of iron sulfides in supporting the terrestrial hot springs origin of life hypothesis.
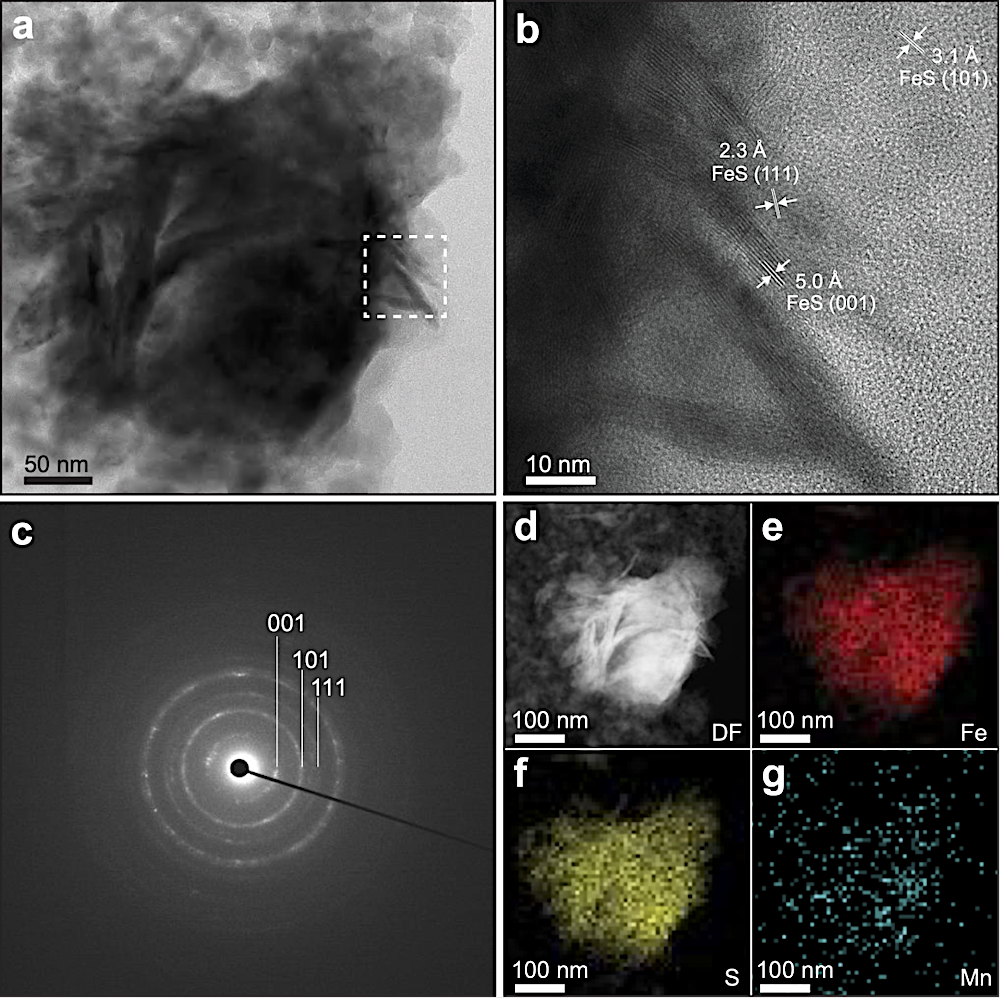
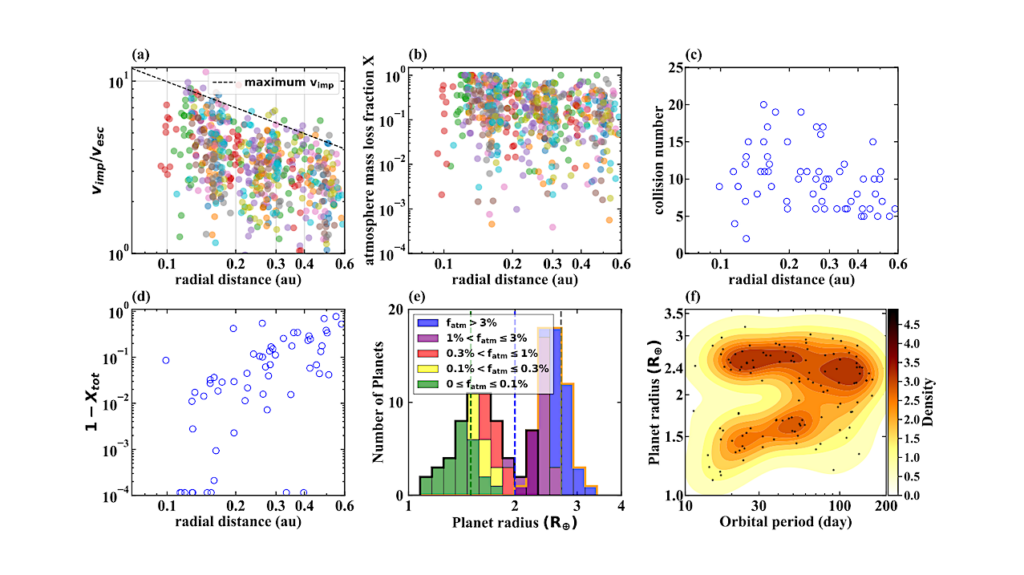
a Bright-field scanning transmission electron microscopy (STEM) image showing the irregular or plate-like crystalline structure of the resultant FeS nanoparticles. b High-resolution TEM (HR-TEM) (enlarged view of area boxed in an image showing lattice fringes of FeS nanoparticles). c The selected area electron diffraction (SAED) pattern showing diffraction rings consistent with mackinawite. d–g STEM image and corresponding energy-dispersive X-ray (EDX) spectroscopy mappings for iron (red), sulfur (yellow), and manganese (blue). DF dark field.
The study was conducted by Dr. NAN Jingbo from the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences, Dr. LUO Shunqin from Japan’s National Institute for Materials Science, Dr. Quoc Phuong Tran from the University of New South Wales, Australia, and other researchers.
Iron sulfides, abundant in early Earth’s hydrothermal systems, may have facilitated essential prebiotic chemical reactions, similar to the function of cofactors in modern metabolic systems. Previous studies on iron sulfides and the origin of life have focused primarily on deep-sea alkaline hydrothermal vents, which provide favorable conditions like high temperature, pressure, pH gradients, and hydrogen (H₂) from serpentinization—factors thought to support prebiotic carbon fixation.
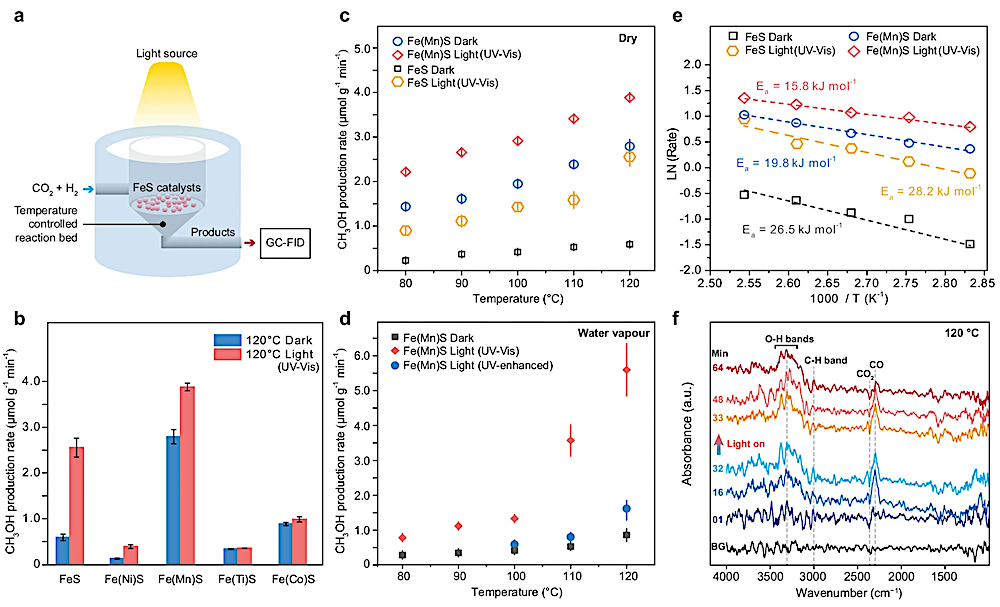
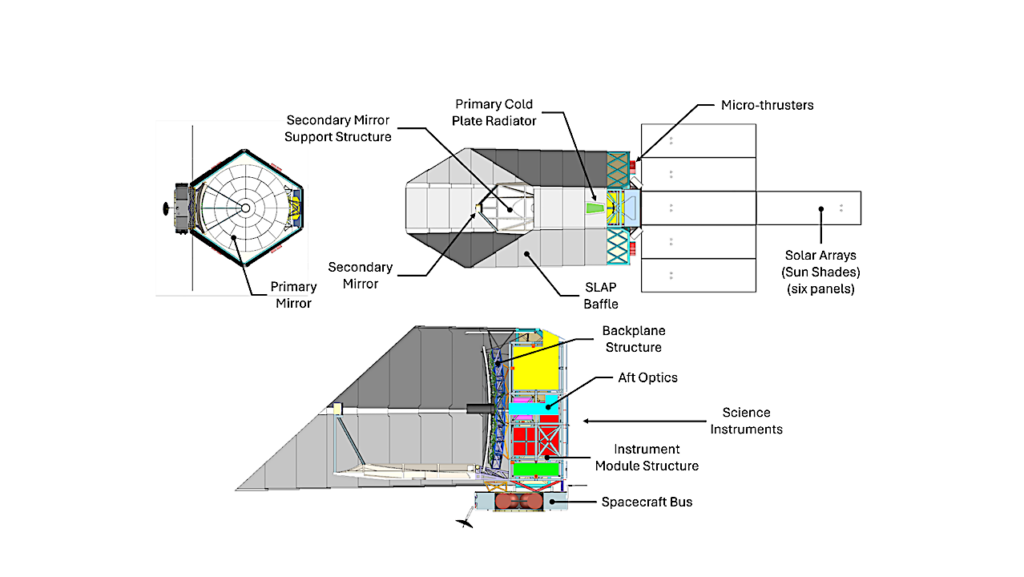
a Schematic representation of the reaction cell used for the light-mediated thermocatalytic reaction. b Effect of metal doping on FeS catalysis under dark and UV–visible light (300–720 nm) conditions. c Temperature-dependent experiments of CH3OH production from CO2 and H2 over pure FeS and Mn-doped FeS under dark and UV–visible light conditions. d Temperature-dependent experiments of CH3OH production in the presence of water vapor over Mn-doped FeS under dark conditions and with the irradiation of UV–visible light or UV-enhanced light (200–600 nm). The error bars in b–d were generated from a triplicate set of measurements. e The apparent activation energies () over FeS and Mn-doped FeS catalysis with and without UV–visible light irradiation. f In situ diffuse reflectance infrared Fourier transform spectra (DRIFTS) of Mn-doped FeS under dark and UV–visible light conditions at 120 °C. Unless otherwise specified, all metal-doped FeS refers to FeS doped with 10% metal. — Nature Communications
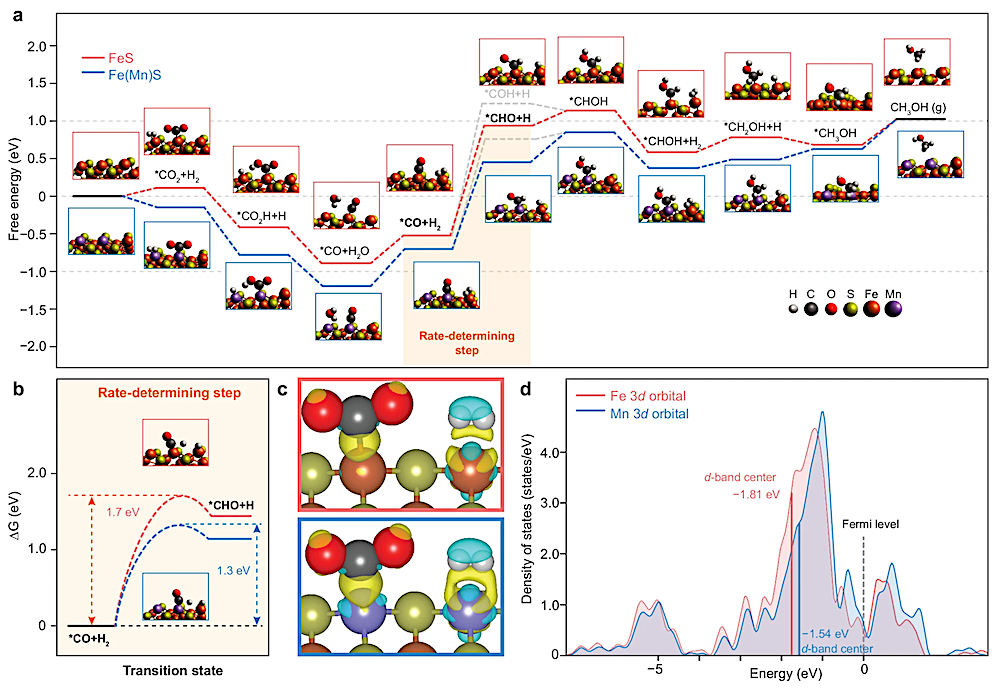
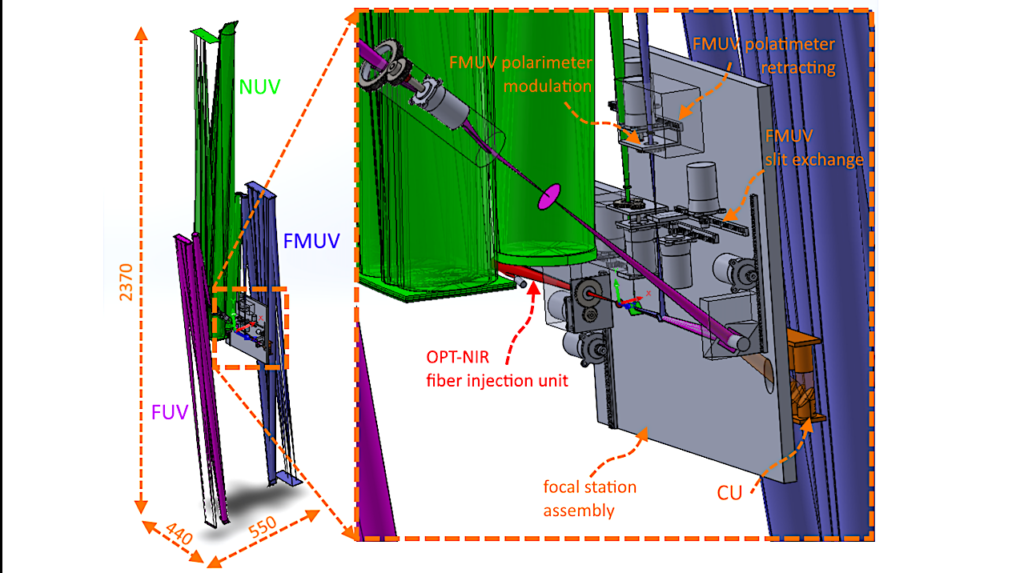
a Gibbs free energy diagram of the reaction steps and corresponding optimized structures. b Transition states of the rate-determining step. c Calculated charge density differences (CDD) between pure FeS (upper) and Mn-doped FeS (lower) upon adsorption of CO2 and H2. Yellow represents electron accumulation, and cyan denotes electron depletion. d Calculated density of states (DOS) of Fe and Mn sites in FeS. — Nature Communications
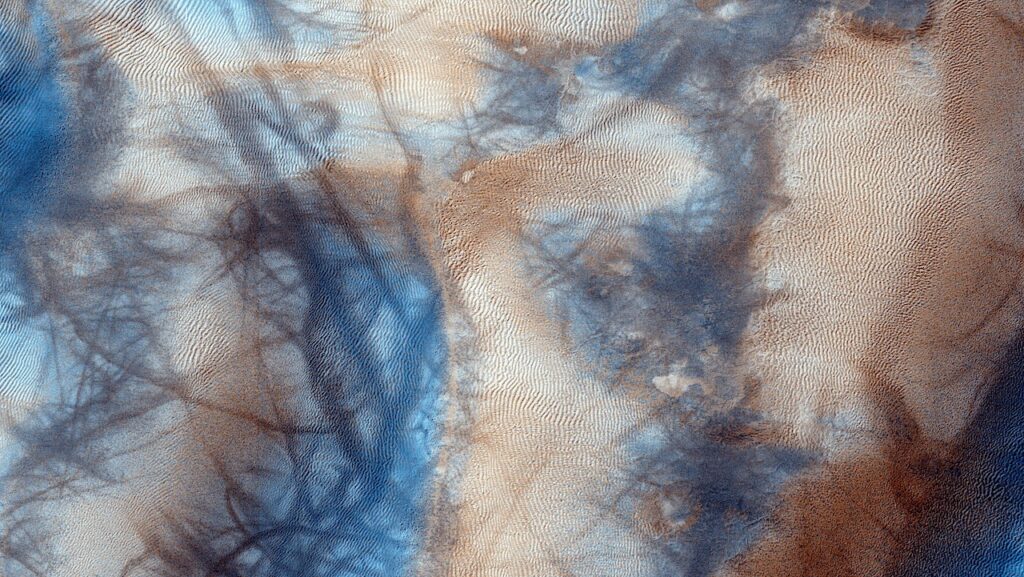
However, some scientists have proposed terrestrial hot springs as another plausible setting for life’s origins, due to their rich mineral content, diverse chemicals, and abundant sunlight (Figure 1).
To explore the role of iron sulfides in terrestrial prebiotic carbon fixation, the research team synthesized a series of nanoscale iron sulfides from mackinawite (Figure 2), including pure iron sulfide and iron sulfides doped with common hot spring elements such as manganese, nickel, titanium, and cobalt.
Their experiments showed that these iron sulfides could catalyze the H₂-driven reduction of CO₂ at specific temperatures (80–120 °C) and atmospheric pressure. Gas chromatography was used to quantify the methanol production (Figure 3).
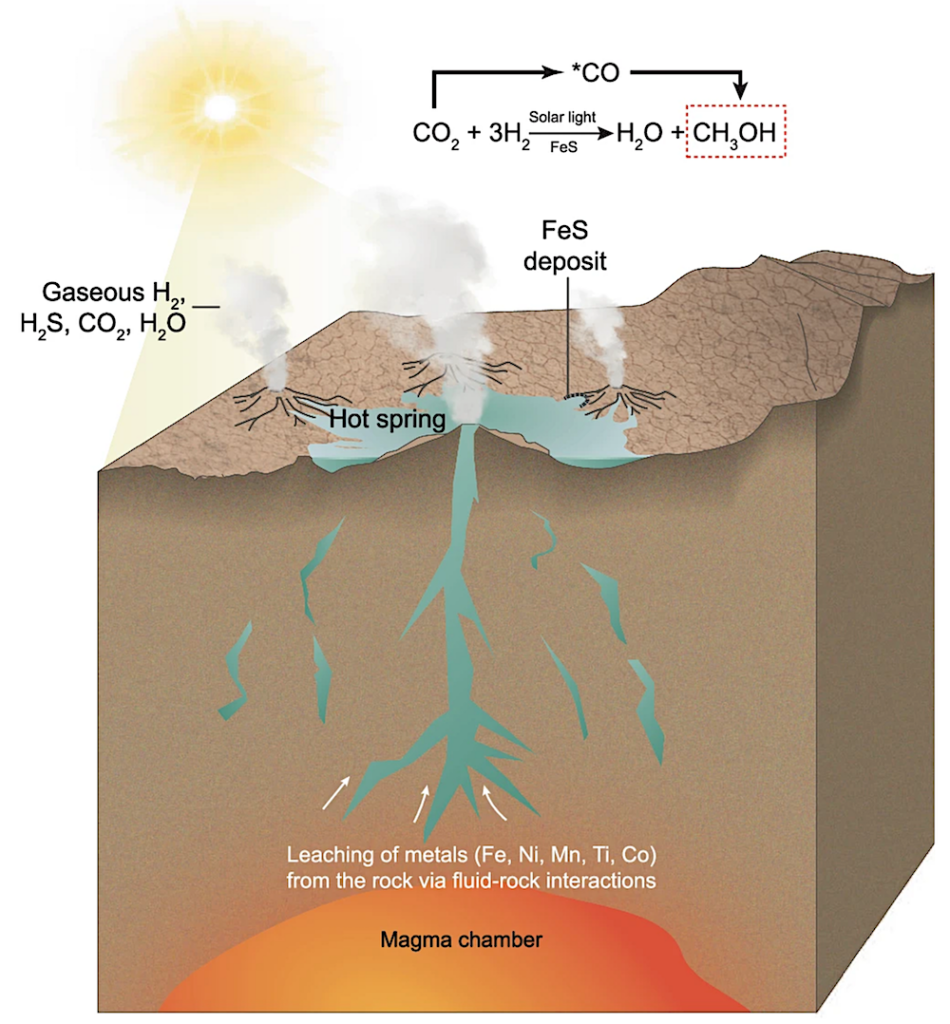
FeS minerals deposited on the edges of hot spring pools catalyze the reduction of CO2 driven by H2 gas released from geochemical processes such as serpentinization. Light irradiation from the young Sun promotes the catalytic activity of FeS. — Nature Communications
The study found that manganese-doped iron sulfides exhibited notably high catalytic activity at 120 °C. This activity was further enhanced by UV-visible (300–720 nm) and UV-enhanced (200–600 nm) light, suggesting that sunlight might play a role in driving this reaction by facilitating chemical processes. Additionally, the introduction of water vapor boosted catalytic activity, further supporting that vapor-laden terrestrial hot springs may have served as key sites for nonenzymatic organic synthesis on early Earth.
To further investigate the mechanism behind the H₂-driven CO₂ reduction, the team conducted in-situ analyses using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS).
Results indicated that the reaction likely proceeds via the reverse water-gas shift (RWGS) pathway, in which CO₂ is first reduced to carbon monoxide (CO), which is subsequently hydrogenated to form methanol. Density functional theory (DFT) calculations provided additional insights, revealing that manganese doping not only lowered the reaction’s activation energy but also introduced highly efficient electron transfer sites, thereby enhancing reaction efficiency (Figure 4). The redox characteristics of iron sulfides make them functionally analogous to modern metabolic enzymes, providing a chemical foundation for prebiotic carbon fixation.
This research underscores the potential of iron sulfides to catalyze prebiotic carbon fixation in early Earth’s terrestrial hot springs, opening new directions for exploring life’s origins and supporting efforts to search for extraterrestrial life.
Iron sulfide-catalyzed gaseous CO2 reduction and prebiotic carbon fixation in terrestrial hot springs, Nature Communications (open access)
Astrobiology