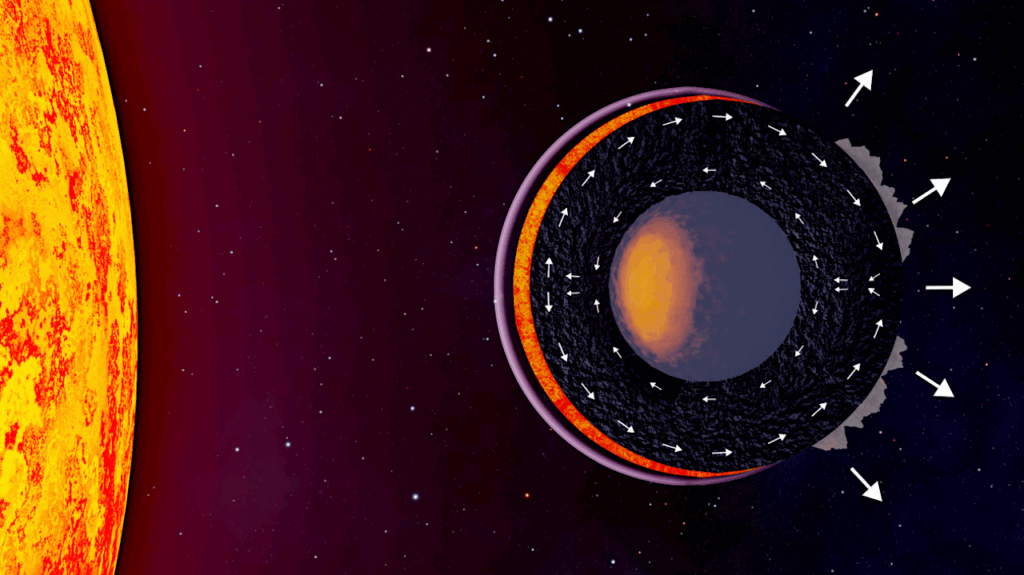
Sulfur on Venus: Atmospheric, Surface, and Interior Processes
Sulfur-bearing species play critical roles in atmospheric physical-chemical processes, atmosphere-surface interactions, and the geological evolution of Venus.
This chapter provides a comprehensive overview of
(1) Instrumental data on the abundance and speciation of sulfur in atmospheric and crustal materials,
(2) the behavior of sulfur-bearing species in the mesosphere, clouds, and lower atmosphere,
(3) chemical and mineralogical aspects of atmosphere-surface interactions,
(4) the fate of sulfur during the formation, differentiation, and geological evolution of Venus, including volcanic degassing, gas-solid reactions at the surface, a putative aqueous period, and subsequent evolution.
The chapter also outlines outstanding questions and discusses further exploration of Venus in the context of sulfur-relevant investigations.
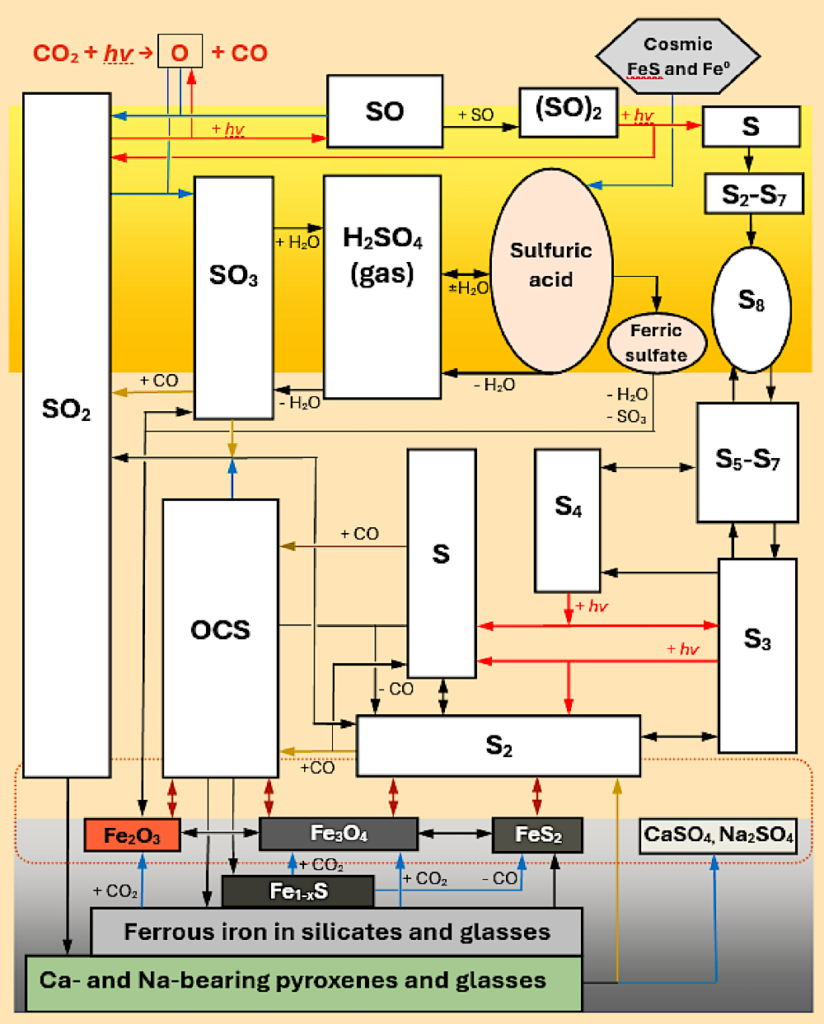
The potential chemical reactions involving S-bearing compounds in the middle and lower atmosphere and in a permeable surface layer on Venus. The orange rectangle represents clouds, and the grey box designates surface materials. The dotted oval signifies near-surface conditions where gas phase and gas-solid type chemical equilibria might occur. White rectangles denote gases, and ovals illustrate aerosol components. Red arrows indicate photochemical reactions. Blue and dark yellow arrows represent oxidation and reduction reactions, respectively. Double arrows illustrate thermochemical equilibria between compounds. Only the significant species and reaction pathways are shown, with details provided in Tables 16.6 and 16.7. H2S, HS, S2O, S-C, and S-Cl-O gases are not depicted. Secondary minerals, such as pyrite, anhydrite, thenardite, magnetite, and hematite, which form via gas-solid reactions, are presented; other secondary phases are discussed in Sect. 16.3.2. Some pathways, such as fates of OCS and Sn and gas-solid reactions that involve Fe sulfides and oxides, are hypothetical. — astro-ph.EP
Abstract
Sulfur-bearing species play critical roles in atmospheric physical-chemical processes, atmospheresurface interactions, and the geological evolution of Venus. Most data on sulfur were obtained for the atmosphere through telescopic and spacecraft (flybys, orbiters) spectroscopic investigations and limited in situ measurements with entry probes.
The atmospheric composition below ~20 km is barely known and is estimated by extrapolating measured contents toward the surface and thermochemical models. The bulk sulfur content was only measured in solid surface samples taken at three landing sites, and the geochemistry of sulfur in the interior is poorly constrained. Sulfur dioxide is the most abundant trace chemically active gas in the middle and lower atmosphere.
Photochemical dissociation of CO2 above clouds to CO and O and subsequent oxidation of SO2 to H2SO4 maintains the existence of thick global clouds rich in sulfuric acid. Interaction of SO2 with basaltic glasses and Ca-bearing minerals leads to the formation of Ca and Na sulfates, consistent with the elevated sulfur content (up to ~2 wt%) in surface probes. Carbonyl sulfide, OCS, is the most abundant reduced atmospheric gas. It forms in the lower atmosphere from CO and sulfur gases (S, S2) and is oxidized by SO3, which forms via pyrolysis of H2SO4 gas below clouds. OCS could be formed and consumed via alteration and formation of sulfide minerals, respectively.
In Hadley cell-type atmospheric circulation, low-latitude upwelling delivers OCS-enriched air toward clouds, and high-altitude downwelling brings OCS-depleted and CO-enriched gas to the deep atmosphere. The occurrence and fate of S1-8 gases and condensates (S8) must be more constrained.
Some models suggest the formation of Sn gases below clouds, and others imply formation via photochemical reactions in upper clouds. Condensation of Sn in clouds remains to be confirmed through observations. S2 is consumed through OCS production in the deep atmosphere and released via silicate sulfatization.
Although chemical disequilibria drive reactions throughout the atmosphere, some gases could equilibrate at the surface. Moreover, the abundance and speciation of S-bearing gases in the deep atmosphere could be controlled by iron sulfide-oxide and/or sulfate-silicate phase equilibria in a permeable surface layer. If this is the case, supplies and sinks of atmospheric sulfur could be compensated by buffering gas-solid reactions. Sulfidation (e.g., pyritization) and sulfatization of surface materials via gas-solid reactions are more thermodynamically favorable at highlands but need confirmation.
Past volcanic degassing of SO2, OCS, S2, H2S, and/or S-Cl gases mainly constituted the current inventory of atmospheric sulfur, and the likelihood of current volcanism is questionable. Although cometary dust is a minor net source of atmospheric sulfur, it could explain elevated mixing ratios of S-bearing gases in the upper mesosphere. Cosmic sources could account for possible metal sulfates in clouds.
Except for trapped atmospheric sulfur, chemical composition and radar-based morphology of immense volcanic formations suggest sulfur abundance and sulfide mineralogy (pyrrhotite, pentlandite) typical for tholeiitic and alkaline basalts. The morphology and thermal emissivity of highly tectonically deformed tessera terrains do not exclude an exposure of rocks formed in water-rich exogenic and/or endogenic environments. Whether sulfates are present in those rocks depends on water history that may or may not involve oxidizing aqueous and/or magmatic environments.
Hydrogen escape from early Venus with putative water ocean could have led to sulfaterich seawater and subsequent sulfate-rich rock formations. Opposite to Earth’s marine sediments, abundant pyrite might not have precipitated in Venus’ counterparts due to the lack of biological sulfate reduction and ample organic matter.
Examining S-bearing species and isotopes should be prioritized in future remote and in situ studies of atmospheric gases, aerosols, and chemically altered and pristine rocks. Upcoming DAVINCI, VERITAS, and EnVision missions will explore atmospheric and surface compositions, allowing a better understanding of the behavior of sulfur on the Earth’s sister planet.
Mikhail Zolotov
Comments: Preprint submitted to “The Role of Sulfur in Planetary Processes: from Atmospheres to Cores”, D. Harlov and G. Pokrovsky (Eds.), Springer Geochemistry. 242 pages, including 13 tables and 31 figures
Subjects: Earth and Planetary Astrophysics (astro-ph.EP)
Cite as: arXiv:2409.13256 [astro-ph.EP] (or arXiv:2409.13256v1 [astro-ph.EP] for this version)
https://doi.org/10.48550/arXiv.2409.13256
Focus to learn more
Submission history
From: Mikhail Zolotov
[v1] Fri, 20 Sep 2024 06:31:18 UTC (4,741 KB)
[v2] Thu, 30 Jan 2025 21:49:11 UTC (6,851 KB)
https://arxiv.org/abs/2409.13256
Astrobiology,