Electricity Driving Life

When nature performs chemical reactions to create energy-rich compounds from simple molecules, it requires energy. So far, it has not been possible to use man-made electricity to drive these biochemical processes.
Researchers at the Max Planck Institute for Terrestrial Microbiology in Marburg have achieved a breakthrough, however: they developed an artificial metabolic pathway that uses electricity to produce ATP, a biochemical energy carrier which can then be used to form energy-rich chemical compounds like starch or protein. The metabolic pathway provides a complete novel approach towards a sustainable, climate-neutral bioeconomy.
Prof. Tobias Erb’s team at the Max Planck Institute in Marburg is currently exploring how synthetic biology can be used to build complex resources from simple molecules. Using an artificial photosynthesis process developed at the Institute, they have already been able to successfully convert carbon dioxide into various valuable resources like antibiotics or biofuels. Their solution thus mimics and improves the way in which photosynthesis in plants converts carbon dioxide.
But just like the natural process it’s trying to improve, synthetic photosynthesis requires energy. The chemical energy currency in nature is adenosine triphosphate (ATP). Its energy is located in the chemical binding: breaking these bonds releases energy that can powers biochemical processes.
ATP via electric current
Sustainable alternatives to fossil fuel is solar or wind energy. But there has never been a way to feed man-made electricity directly into biochemical reactions.
Tobias Erb and his team have now developed an enzyme cascade that can produce ATP via electric current. The enzymatic cascade referred to as the “AAA cycle” consists of four biocatalysts. The first and main enzyme, aldehyde ferredoxin oxidoreductase (AOR), reduces an acid to an aldehyde. “The electrical energy is stored in the aldehyde bond. The remaining three enzymes are responsible for the regeneration of the aldehyde. This process releases energy that is used to generate ATP, ” explains Shanshan Luo, lead author of the study. The ATP from the AAA cycle can be used to power chemical reactions, like the production of glucose-6-phosphate, the building block for starch. It can also be used for protein synthesis.
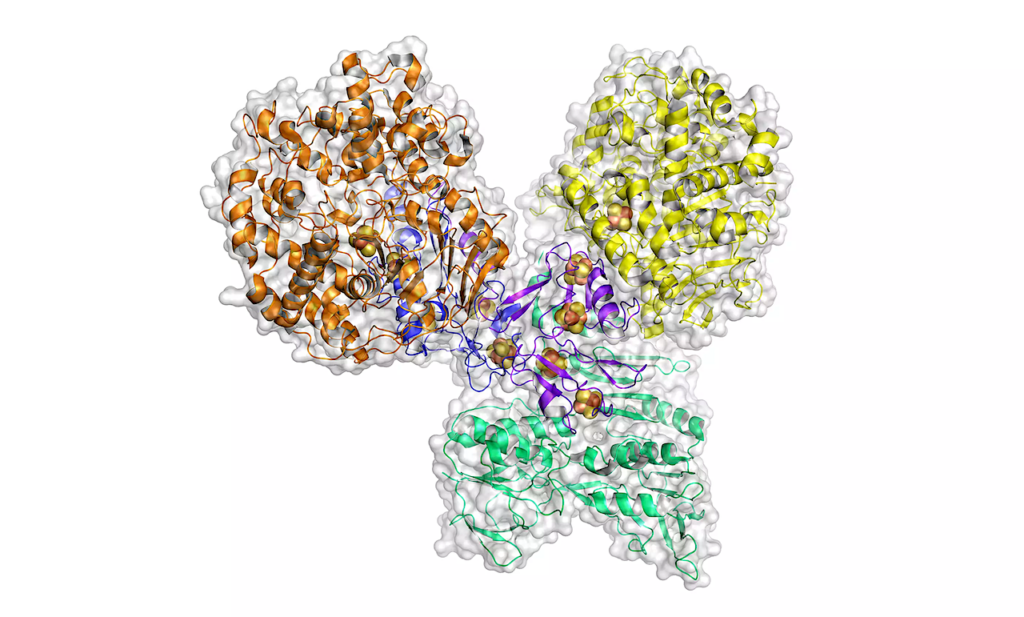
A 3D structural model of the enzyme aldehyde ferredoxin oxidoreductase (AOR). © Max Planck Institute for Terrestrial Microbiology/Erb
The researchers discovered the AOR in a scientifically still poorly known bacterium called Aromaticum aromatoleum. Researchers at the Center for Synthetic Microbiology at the University of Marburg were able to cultivate the microbe under oxygen-free lab conditions to study its ability to degrade petroleum in nature. Now this serendipitous discovery forms the core of the AAA cycle.
Interface between electricity and biology
“It has never been possible to power ATP-dependent biochemical reactions with electricity. The AAA cycle is now able to directly convert electrical energy into biochemical energy,” says Tobias Erb, Director at the Max Planck Institute for Terrestrial Microbiology. “This will enable synthesis of energy-rich valuable resources such as starch, biofuels or proteins from simple cellular building blocks –in the future even from carbon dioxide. It may even be possible to use biological molecules to store electrical energy.
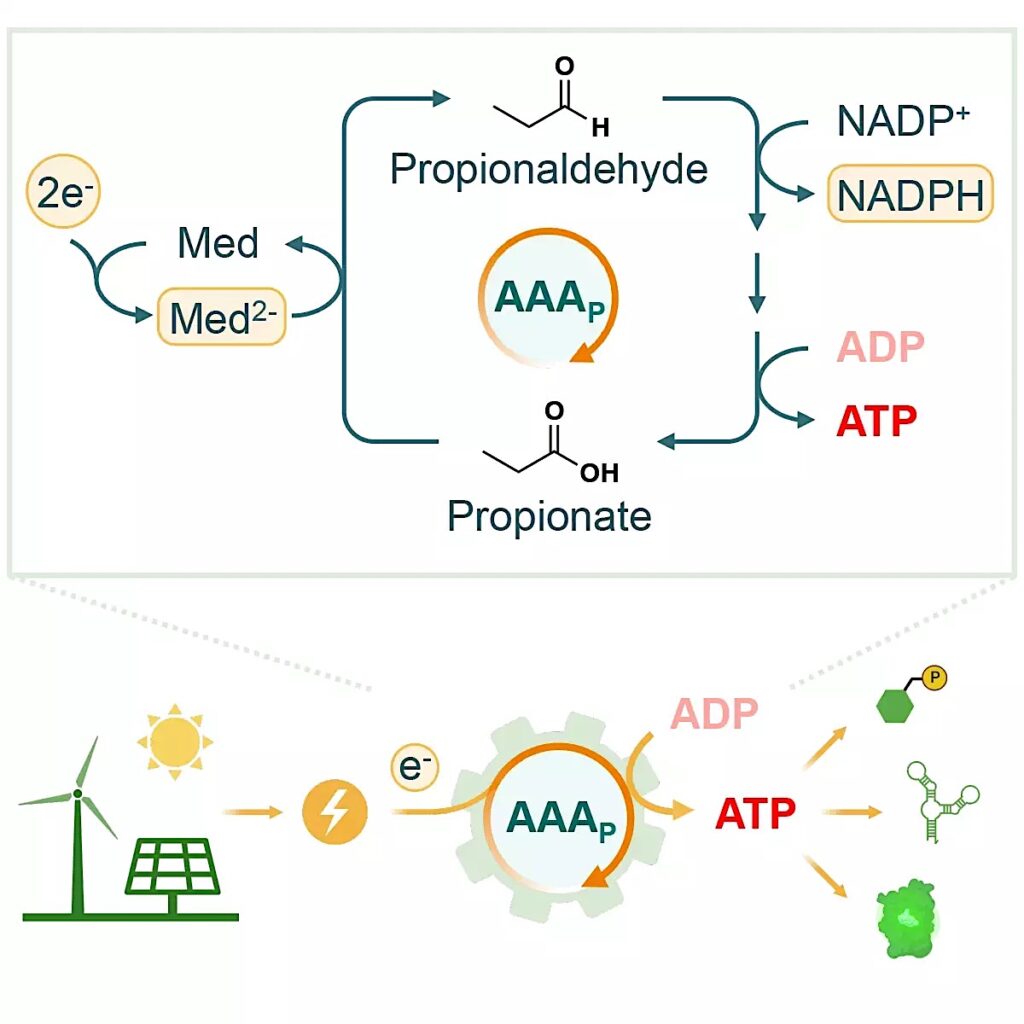
AAA-Cycle. Max Planck Institute for Terrestrial Microbiology/Erb
Extensive research is still needed before the new proof-of-concept can be used in practical applications, however. The enzymes still lack stability and break down when exposed to oxygen. Currently, only small amounts of energy are converted. So before this innovation can be used on an industrial scale, researchers have a lot of work to do. “In the future, it may be possible for the AAA cycle to operate at the interface between electricity on the one hand and biology on the other. Feeding electricity directly into chemical and biochemical reactions is a real breakthrough, however” says Erb.
The research project is part of eBioCO2, a joint project of Max Planck Society and Fraunhofer-Gesellschaft. The aim of the project is to transfer knowledge from basic research to the development of new technologies.. The project also received support from the German government’s GreenTalent programme.
ATP production from electricity with a new-to-nature electrobiological module, Cell (open source)
Astrobiology