Revisiting The Reactivity Between HCO and CH3 On Interstellar Grain Surfaces
Formation of interstellar complex organic molecules is currently thought to be dominated by the barrierless coupling between radicals on the interstellar icy grain surfaces.
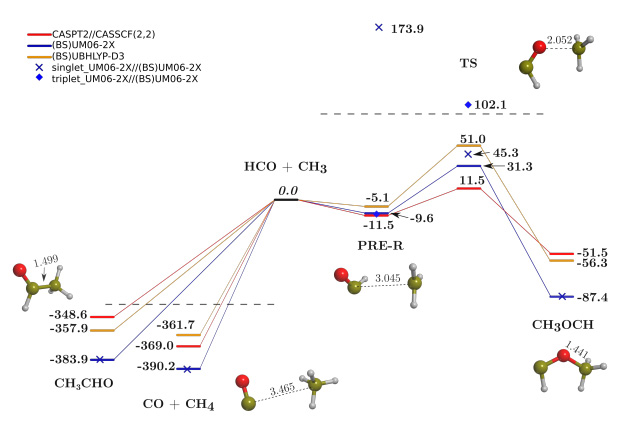
Previous standard DFT results on the reactivity between CH3 and HCO on amorphous water surfaces, showed that formation of CH4 + CO by H transfer from HCO to CH3 assisted by water molecules of the ice was the dominant channel. However, the adopted description of the electronic structure of the biradical (i.e., CH3/HCO) system was inadequate (without the broken-symmetry (BS) approach). In this work, we revisit the original results by means of BS-DFT both in gas phase and with one water molecule simulating the role of the ice.
Results indicate that adoption of BS-DFT is mandatory to describe properly biradical systems. In the presence of the single water molecule, the water-assisted H transfer exhibits a high energy barrier. In contrast, CH3CHO formation is found to be barrierless. However, direct H transfer from HCO to CH3 to give CO and CH4 presents a very low energy barrier, hence being a potential competitive channel to the radical coupling and indicating, moreover, that the physical insights ofthe original work remain valid.
J. Enrique-Romero, S. Álvarez-Barcia, F. J. Kolb, A. Rimola, C. Ceccarelli, N. Balucani, J. Meisner, P. Ugliengo, T. Lamberts, J. Kästner
(Submitted on 14 Feb 2020)
Comments: Submitted to MNRAS main journal. For associated supporting material refer to the publication in MNRAS. Accepted 2020 February 14. Received 2020 February 14
Subjects: Astrophysics of Galaxies (astro-ph.GA); Chemical Physics (physics.chem-ph)
Cite as: arXiv:2002.06101 [astro-ph.GA] (or arXiv:2002.06101v1 [astro-ph.GA] for this version)
Submission history
From: Joan Enrique-Romero
[v1] Fri, 14 Feb 2020 16:11:44 UTC (1,209 KB)
https://arxiv.org/abs/2002.06101
Astrobiology, Astrochemistry








